April 13, 2020
Alberta study investigates the impact of early intervention with hydroxychloroquine to help patients battling COVID-19

May 26, 2020 - Out of an abundance of caution for the safety of our study participants we have temporarily suspended enrolment into the Alberta HOPE COVID-19 Trial into the effectiveness of hydroxychloroquine (HCQ) as an early intervention for COVID-19 until we can fully consider the results from this study and others that are ongoing. Followup and monitoring of patients enrolled in the trial will continue.
University of Calgary and University of Alberta researchers are leading a province-wide study to investigate the effectiveness of the well-tolerated drug hydroxychloroquine (HCQ) as an early intervention for Albertans who test positive for COVID-19. Treatment options for COVID-19 are unclear and research is critical to finding new avenues to help people battle the virus.
With support from Alberta Health Services Strategic Clinical Networks, and the Government of Alberta, the randomized, double-blind, placebo-controlled clinical trial Alberta HOPE COVID-19 will recruit over 1,600 Albertans who have recently tested positive for COVID-19 to determine whether a prescribed five-day treatment of HCQ can prevent hospitalization for those at highest risk of developing a severe illness.
“We will be targeting Albertans who have an underlying medical condition which has proven to contribute to the worsening of symptoms, and eventual hospitalization,” says Dr. Luanne Metz, MD, principal investigator on the study, acting facility medical director, Foothills Medical Centre, and professor at the Cumming School of Medicine (CSM). “Managing Albertans entirely in the community will ensure that hospital resources are available for those who are severely ill and others who require medical attention for conditions other than COVID-19.”

Dr. Luanne Metz, MD (trial lead) and Dr. Michael Hill, MD (co-lead)
Study focuses on Albertans who test positive
The study will recruit Albertans who are at home, can swallow a pill and can begin the treatment protocol within 96 hours of testing positive for the SARS-CoV-2, the virus that causes COVID-19. They also have to be within a 12-day window of the first reported symptoms of the virus.
“Alberta HOPE COVID-19 is an Alberta-only unique trial, designed, run and funded in Alberta,” says Dr. Michael Hill, MD, study co-lead in Calgary and professor at the CSM. “The clinical trials team at the university and AHS are highly experienced and dedicated to this effort, thanks to our proven experience we were able to get this trial up and running at an unprecedented pace.”
The drug and placebo for the trial are being donated by Canadian manufacturer, Apotex. HCQ is originally an anti-malarial drug currently used mostly for immunological disorders like rheumatoid arthritis. Laboratory studies also suggest that it may be helpful against the COVID-19 virus, however, there have only been very small studies suggesting clinical benefit.
“Clinical trials like this will give health-care professionals more evidence to determine how best to care for patients,” says Dr. Kathryn Todd, PhD, AHS vice-president, provincial clinical excellence. “AHS is rallying alongside its academic partners at the universities of Calgary and Alberta to help leverage research in the response to this global pandemic.”
The study process
Staff from Alberta Health Services will obtain permission from individuals with positive COVID-19 tests to provide their contact information to the researchers. Consenting participants will be screened for safety and eligibility. The treatment will then be delivered to their door, anywhere in Alberta. Receipt of the treatment will be witnessed by the courier and confirmed by phone. Participants will then be followed up by phone on day seven and day 30 of starting the treatment.
The study is supported by Calgary Health Trust, Alberta Innovates, the Government of Alberta and the University of Calgary/Alberta Health Services Clinical Research Fund.
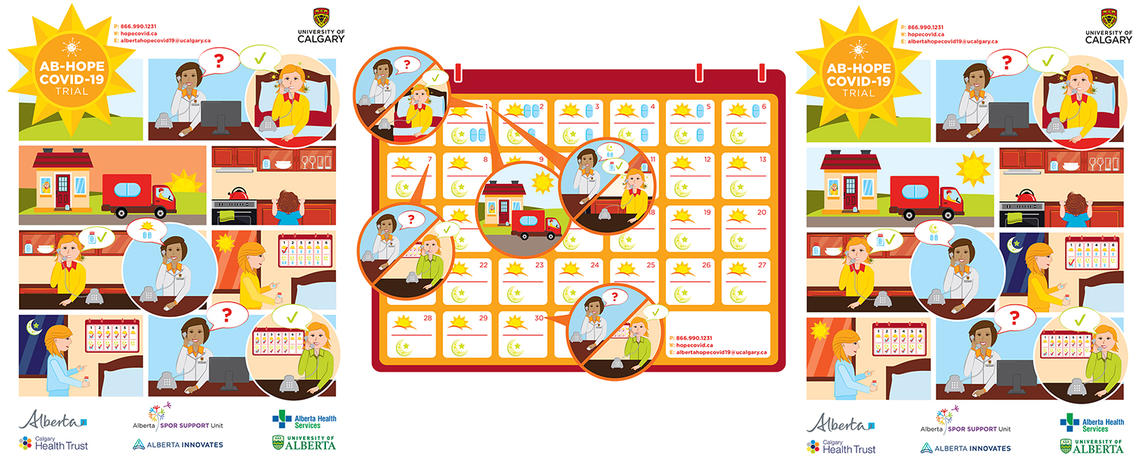
AB-HOPE COVID-19 Trial Steps
Related but different study
Researchers are seeking approval to ask participants in the Alberta HOPE COVID-19 trial whether other household members, who have not tested positive for COVID-19, may be interested in joining a different research study. U of A researchers, Dr. Lawrence Richer, MD, and Dr. Ilan Schwartz, MD, PhD, are investigating whether HCQ may be effective in slowing or even preventing the transmission of COVID-19.
“This prevention trial is for people who are exposed to somebody infected with COVID-19,” says Schwartz, an infectious disease specialist and U of A site-lead for the clinical trial. “Eligible participants will be adults — who don't have symptoms and have not tested positive for COVID-19 — who live with someone diagnosed with COVID-19, and health-care workers who have exposure to known cases.”
That study will look at whether the medication, when administered within a few days of a person’s first exposure, can prevent infection from occurring and whether it can prevent progression to a disease requiring hospitalization. Half the participants will receive HQC the other half will receive a placebo.
To find out more about these studies and other research projects related to COVID-19 that you can participate in, check out the Be the Cure website.
Dr. Luanne Metz, MD, is a neurologist, and the acting facility medical director at the Foothills Medical Centre. She is a professor in the Department of Clinical Neurosciences at the CSM and member of the Hotchkiss Brain Institute and O’Brien Institute for Public Health.
Dr. Michael Hill, MD, is a neurologist and clinical trialist at the Foothills Medical Centre and a professor in the Cumming School of Medicine’s departments of Clinical Neurosciences, Radiology, Medicine, and Community Health Sciences and a member of the Hotchkiss Brain Institute, the Libin Cardiovascular Institute and the O’Brien Institute for Public Health at the University of Calgary.
Dr. Ilan Schwartz, MD, PhD, is an infectious diseases specialist and an assistant professor of medicine at the University of Alberta's Faculty of Medicine and Dentistry.
Dr. Lawrence Richer MD, MSc, is director of the Northern Alberta Clinical Trials and Research Center, associate dean of Clinical Research, and associate director of the Women and Children's Health Research Institute. He is a pediatric neurologist and professor in the Department of Pediatrics at the University of Alberta.
UCalgary resources on COVID-19
For the most up-to-date information about the University of Calgary's response to the spread of COVID-19, visit the UCalgary COVID-19 Response website.
For resources to support students, faculty, staff, alumni, and all our communities during this unprecedented time, visit the UCalgary COVID-19 Community Support website.
